Your Path to Technical Documentation
Comprehensive, innovative, tailored and global
As regulatory requirements continue to increase, the working environment becomes ever more volatile. Topics like collaboration, expanding teams, and cross-location workflows are now everyday challenges for medical device manufacturers. This is precisely why BAYOOSOFT Themis is redefining the path to Technical Documentation – holistically, innovatively, tailored to your needs, and on a global scale.

The Guideline for Technical Documentation
✓ What documents need to be submitted to the Notified Body for approval?'
✓ Has the provided information been reviewed, approved, or flagged for further action??
✓ Who is working on it, and what still needs to be approved?
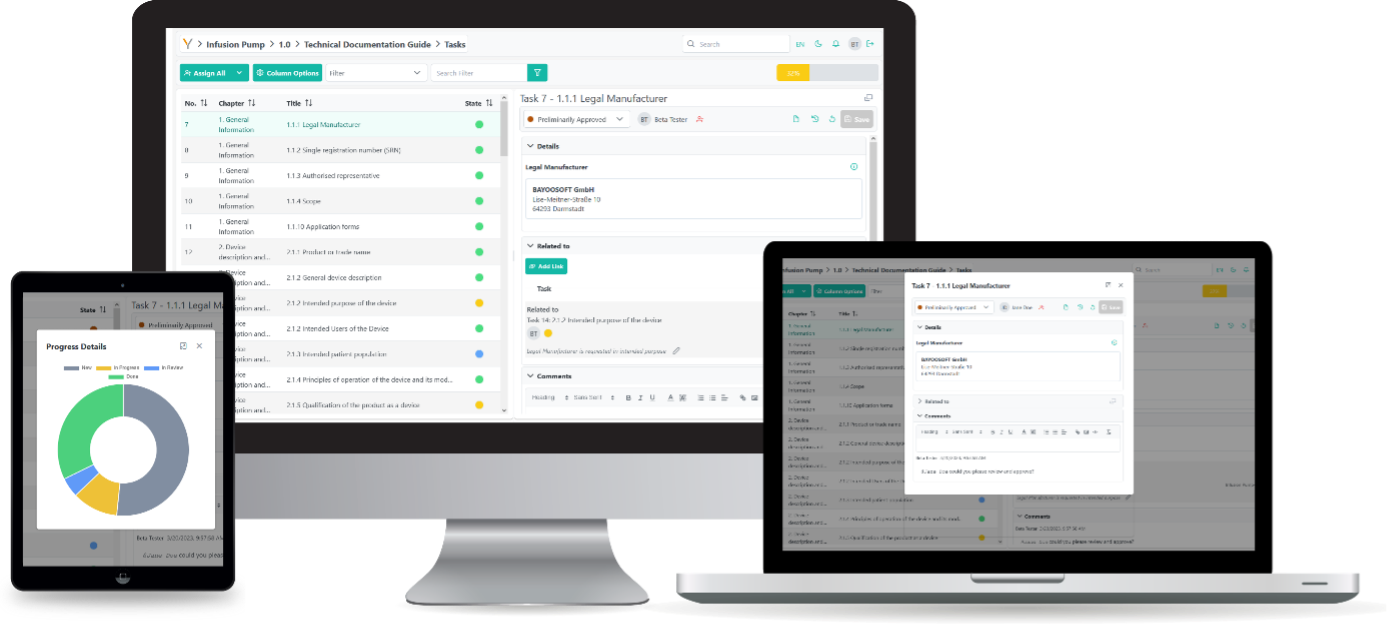
Gain full transparency and obtain a complete overview with the Themis MDR/IVDR Documentation Guide.

Teamwork
Collaboration Rethought
Thanks to multi-user capability, colleagues can simultaneously capture content and results in the same document and are guided through the process in a structured manner. The use of task labelling and assignment (for open and processed tasks), team dashboards, notifications, as well as chats and comments, enables easy and fast communication within the team – even across multiple locations.




Digitization
A Centralized Data Pool
In a clearly structured environment, all relevant information is captured and dynamically linked in fine detail. The knowledge database creates cross-project synergies and promotes company-wide utilization of the developed know-how. The design of the knowledge platform and structured templates enable efficient and secure execution of documentation processes.
Compliance
In conformity with MDR & IVDR
The approval of your product by notified bodies largely depends on compliance with the required guidelines, standards, and regulations.
Our software solution creates complete and compliant technical documentation from your data and efficiently guides your team through all necessary processes, even if team members have no prior experience.


* The product "Authorization Fee for Payment Method" will be added to your shopping cart, with a fee of €0.50 (excluding VAT).
For the downloadable version (on-premise installation), please contact our sales department at: [email protected]
|
|
Cloud Hosted / Software as a Service |
|
Fulfilled Regulatory Requirements |
MDR 2017/745 |
|
Document Management |
✓ |
|
Traceability |
✓ |
|
Generation of Technical Documentation Files including folder structure |
✓ |
|
Team Collaboration Capabilities |
✓ |
|
Versioning & Approval Workflow |
✓ |
|
Audit Trail & Version History |
✓ |
|
Validated according to ISO 13485 |
Environment validated by the manufacturer |
|
Technical Support |
✓ |
|
Optional Services |
30-Day Free Trial Period* |
* The product "Authorization Fee for Payment Method" will be added to your shopping cart, with a fee of €0.50 (excluding VAT).
The licensing of the Themis MDR/IVDR Guide is done in the form of Named-User licenses, which can be assigned directly by the system administration in the software solution after purchase. The minimum order quantity is three Named-User licenses.
Licenses are available on a subscription basis with monthly or annual terms, with the first month offered as a free trial period.
* The product "Authorization Fee for Payment Method" will be added to your shopping cart, with a fee of €0.50 (excluding VAT).

 DE
DE 
