Your requirements efficiently
Requirements analysis is an indispensable part of the efficient and user-friendly development of complex medical device systems. It is particularly important for interrelated product and system concepts and development based on division of labour. By recording, specifying and validating the requirements, potential sources of error can be identified at an early stage and proactively addressed. The results are improved product quality, shorter development times and ultimately cost savings.
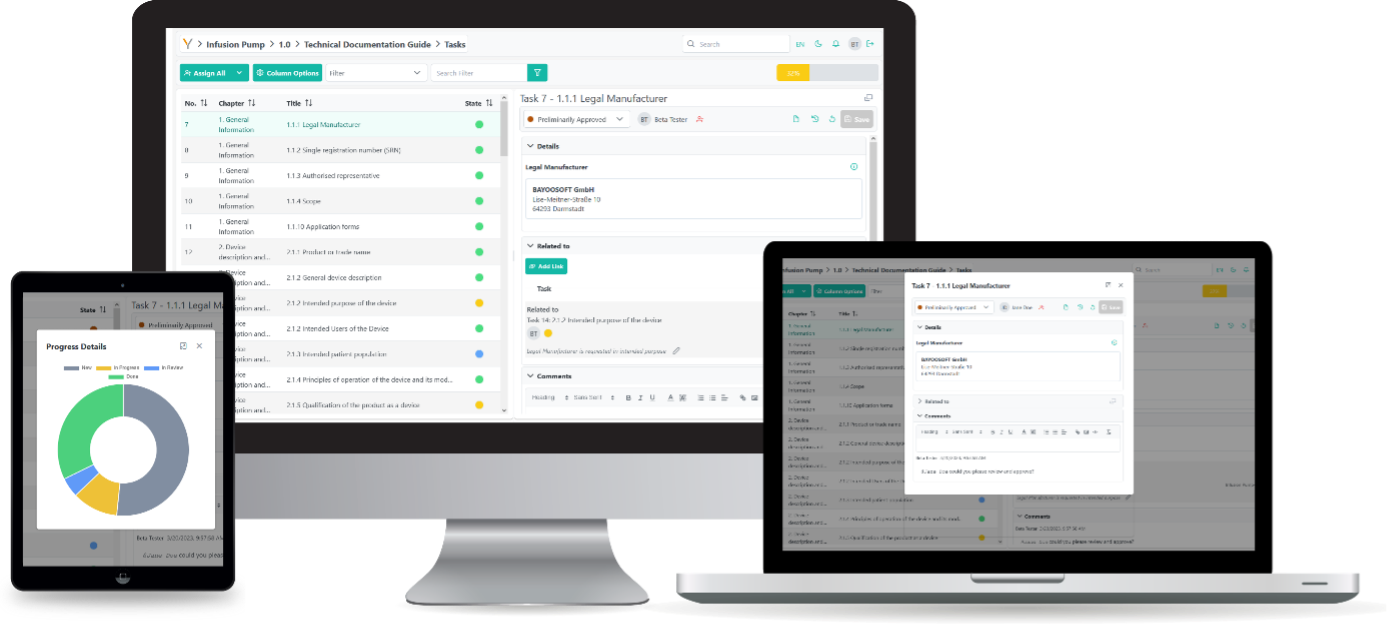
This requirements analysis supports you in documenting the requirements comprehensively and comprehensibly throughout the product life cycle.

Essentials Principals Checklist: Essential safety and performance requirements
In order to be able to place your medical device or in-vitro diagnostic medical device on the market, in addition to the fulfilment of product-specific requirements, conformity with the essential safety and performance requirements or essential principles of the target countries in particular must be demonstrated. The "Essential Principles Checklist" module contained in the Requirements Pack offers you an interactive checklist with which you only have to answer the relevant questions for the product. This allows you to fulfil the same requirements for different target countries only once for your product but generate the necessary reports individually for each country. The following areas of application are currently available:
✓ European Union (medical devices and in-vitro diagnostics) / MDR & IVDR
✓ MDSAP countries
- Australia
- Canada
Checklist for compliance with the Essential Health and Safety Requirements according to (EU) Regulation 2023/1230 on machinery
Regulation (EU) 2023/1230 has been applicable to the manufacture and placing on the market of machinery in Europe since June 14, 2023. This is aimed at manufacturers who have to guarantee the requirement for safety in the interaction between man and machine. Annex III of this regulation defines essential health and safety requirements to ensure a high level of health and safety protection.
With BAYOOSOFT Themis, you carry out the conformity assessment and document the applicability of each requirement and the methods. You can store the norms and standards you have applied and output the results as a complete report.
Checklist IEC 60601-1 Medical electrical equipment
By complying with the IEC 60601-1 standard, medical device manufacturers ensure the basic safety and essential performance characteristics of medical electrical equipment and medical electrical systems. In order to provide documented proof of conformity for medical devices, you now have the opportunity to record the applicability of the numerous requirements and the methods for verification with BAYOOSOFT Themis.

Teamwork
Collaboration Rethought
Thanks to multi-user capability, colleagues can simultaneously capture content and results in the same document and are guided through the process in a structured manner. The use of task labelling and assignment (for open and processed tasks), team dashboards, notifications, as well as chats and comments, enables easy and fast communication within the team – even across multiple locations.




Digitization
A Centralized Data Pool
In a clearly structured environment, all relevant information is gathered and dynamically linked with each other in a fine-granular manner. The knowledgebase also generates cross-project synergies and enables you to use the generated know-how throughout the organization. The creation of a knowledge platform as well as structured templates facilitates the efficient and secure performance of documentation processes.
Compliance
In Conformity to ISO Norm
The approval of your product by the Notified Body will be determined by its conformity with the applicable directives, norms and standards. The software solution generates comprehensive and compliant Technical Documentation based on your data and guides your team efficiently through all the necessary processes – even for inexperienced colleagues.


* The product "Authorization Fee for Payment Method" is added to your shopping cart and we charge a fee of €0.50 (excl. VAT) for this.
For the downloadable version (on-premise installation), please contact our sales department at: [email protected]
|
|
Cloud hosted / Software as a Service |
|
Modules included in the package |
Requirements Engineering |
|
Document Management |
✓ |
|
Traceability |
✓ |
|
Generation of Technical Documentation Files including folder structure |
✓ |
|
Team Collaboration Capabilities |
✓ |
|
Versioning & Approval Workflow |
✓ |
|
Audit Trail & Version History |
✓ |
|
Validated according to ISO 13485 |
Environment is validated by manufacturer |
|
Technical Support |
✓ |
|
Optional Services |
30-Day Free Trial Period* |
* The product "Authorization Fee for Payment Method" is added to your shopping cart and we charge a fee of €0.50 (excl. VAT) for this.
Themis Requirements Pack is licensed in the form of named user licenses, which can be assigned directly in the software solution by the system administrator after purchase. The minimum purchase quantity is three named users.
Licenses can be purchased in the form of a subscription model with a monthly or annual term. The first month is a free trial period.
* The product "Authorization Fee for Payment Method" will be added to your shopping cart, with a fee of €0.50 (excluding VAT).

 DE
DE 
