A new approach to technical documentation
Efficient and Compliant
Your validated solution for documentation processes of medical devices and in-vitro diagnostics – digitize interconnected processes and sustainably reduce documentation efforts by minimizing redundant data.
With increasing regulatory requirements and an increasingly volatile work environment, manufacturers of medical devices and in-vitro diagnostics face new challenges. Topics such as collaboration, growing teams, and cross-location work have become everyday realities in the world of medical device and in-vitro diagnostic manufacturers.
How can you keep efforts and costs as low as possible?
With BAYOOSOFT Themis, the validated and compliant solution for boosting regulatory productivity.
Here’s how it works: Processes are digitized and accelerated through the centralized management and linking of individual information units. This not only optimizes individual tasks but entire process chains, reducing resource usage and minimizing error rates compared to manual processing. As a result, operational efforts, such as updating existing files, are sustainably reduced. At the same time, you enhance the acceptance of your (technical) documentation with your Notified Body.
Themis Ultimate includes all Themis Guides as well as all available Themis Packs for the MedTech industry and IT security, offering you an ideal solution for all functions within your organization.
Themis Ultimate includes:
- MDR&IVDR Documentation Guide
- Browser-based guideline for creating technical documentation.
- ISO 27001 Guide
- ISO 9001 Guide
- ISO 13485 Guide
- Medical Risk Pack
- Risk Management according to ISO 14971
- Requirements Pack
- Requirements Engineering:
This requirements analysis supports you in documenting the requirements comprehensively and comprehensibly throughout the product life cycle. - Essentials Principles Checklist:
With Essential Principles Checklist, you can record your requirements for various target countries using an interactive checklist.
- Requirements Engineering:
- Management Systems Pack
- Digital Management of your Quality / Information Security Management System


Teamwork
Collaboration Rethought
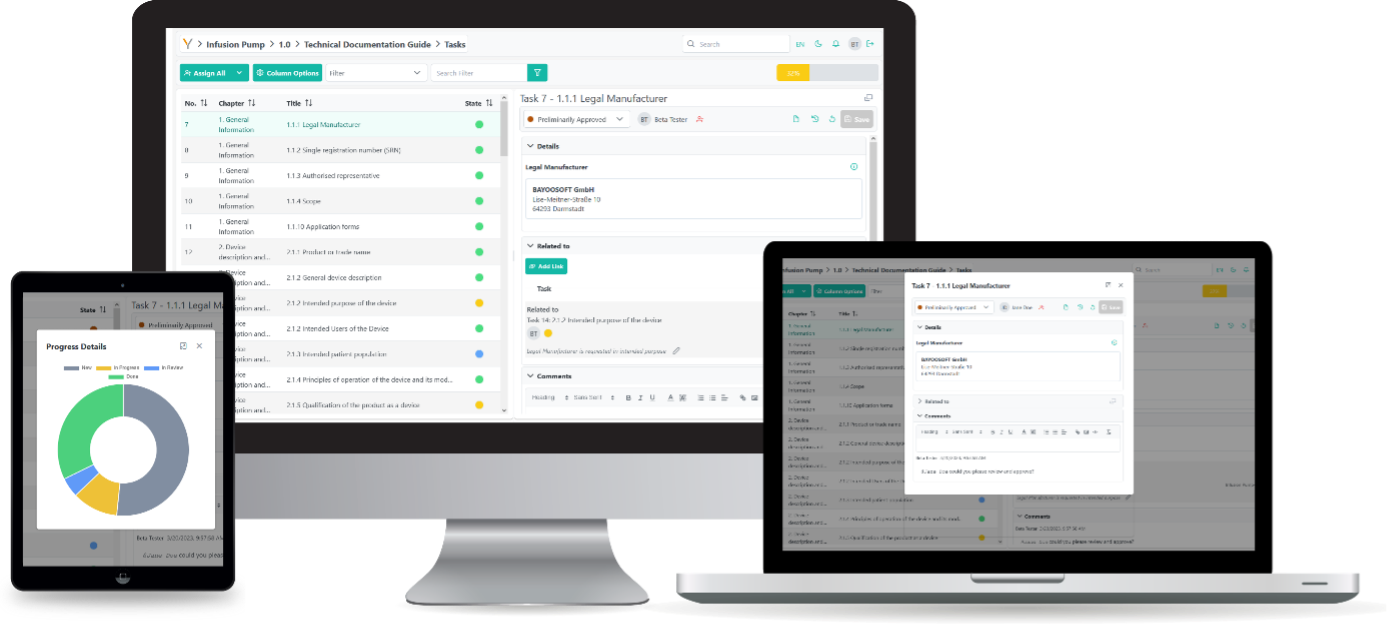
Thanks to multi-user capability, colleagues can simultaneously capture content and results in the same document and are guided through the process in a structured manner. The use of task labelling and assignment (for open and processed tasks), team dashboards, notifications, as well as chats and comments, enables easy and fast communication within the team – even across multiple locations.




Digitization
A Centralized Data Pool
In a clearly structured environment, all relevant information is gathered and dynamically linked with each other in a fine-granular manner. The knowledgebase also generates cross-project synergies and enables you to use the generated know-how throughout the organization. The creation of a knowledge platform as well as structured templates facilitates the efficient and secure performance of documentation processes.
Compliance
In Conformity to Several ISO norms
The approval of your product by notified bodies largely depends on compliance with the required guidelines, standards, and regulations.
Our software solution creates complete and compliant technical documentation from your data and efficiently guides your team through all necessary processes, even if team members have no prior experience.


* The product "Authorization Fee for Payment Method" will be added to your shopping cart, with a fee of €0.50 (excluding VAT).
For the downloadable version (on-premise installation), please contact our sales department at: [email protected].
|
|
Cloud hosted |
|
Modules included in the package |
All modules of the following Guides and Packs:
|
|
Document Management |
✓ |
|
Traceability |
✓ |
|
Generation of Documentation |
✓ |
|
Team Collaboration Capabilities |
✓ |
|
Versioning & Approval Workflow |
✓ |
|
Audit Trail & Version History |
✓ |
|
Validated according to ISO 13485 |
Environment is validated by manufacturer |
|
Technical Support |
✓ |
|
Optional Services |
30 days free trial period* |
* The product "Authorization Fee for Payment Method" will be added to your shopping cart, with a fee of €0.50 (excluding VAT).
Themis Ultimate is licensed in the form of named user licenses, which can be assigned directly in the software solution by the system administrator after purchase. The minimum purchase quantity is three named users.
Licenses can be purchased in the form of a subscription model with a monthly or annual term. The first month is a free trial period.
* The product "Authorization Fee for Payment Method" will be added to your shopping cart, with a fee of €0.50 (excluding VAT).

 DE
DE 
